Tamoxifen⁚ A Comprehensive Overview
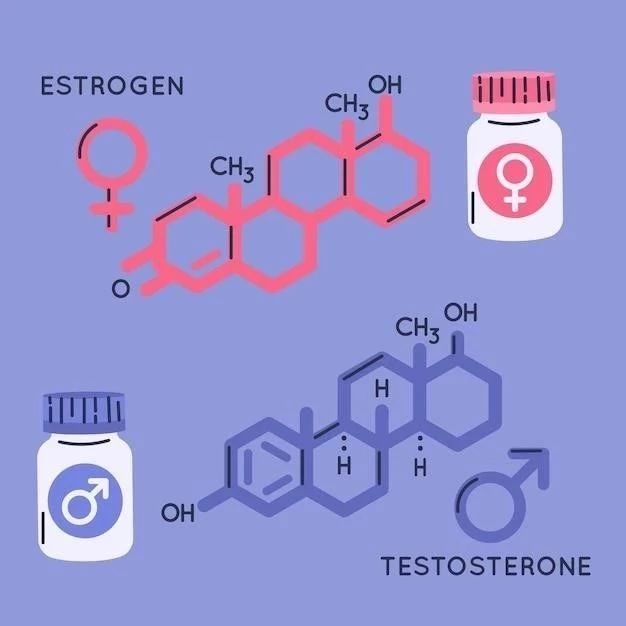
Tamoxifen, a selective estrogen receptor modulator (SERM), displays a dual mechanism of action. It competitively inhibits estrogen binding to its receptor, thereby hindering the growth of estrogen-receptor-positive breast cancer cells. This action also influences tumor growth factors and sex hormone-binding globulin levels, impacting overall tumor development.
Introduction to Tamoxifen
Tamoxifen, a widely prescribed medication, holds a significant role in managing and preventing certain types of breast cancer. Its classification as a selective estrogen receptor modulator (SERM) highlights its unique interaction with estrogen receptors within the body. Unlike estrogen, which promotes the growth of some breast cancer cells, Tamoxifen acts as a competitive inhibitor. It binds to these same receptors, effectively preventing estrogen from binding and thus halting or slowing the growth of estrogen-receptor-positive breast cancer. This intricate interaction forms the basis of its therapeutic effect. Understanding Tamoxifen’s mechanism of action is crucial for healthcare professionals to effectively utilize it in treatment plans. Its complex effects extend beyond simple antagonism, impacting various physiological processes and influencing overall treatment outcomes. Detailed knowledge of its multifaceted effects allows for personalized patient care and informed decision-making regarding its use.
Tamoxifen’s Mechanism of Action⁚ A Detailed Look
Tamoxifen’s primary mechanism involves its interaction with estrogen receptors (ERs), specifically ERα, found within breast cancer cells. It acts as a competitive inhibitor, meaning it vies with estrogen (estradiol) for binding sites on the ER. When Tamoxifen successfully binds, it prevents estrogen from initiating its growth-promoting effects on cancer cells. This competitive binding is not uniform across all tissues; Tamoxifen exhibits tissue-specific effects, acting as an agonist (mimicking estrogen) in some tissues and an antagonist (blocking estrogen) in others. This unique characteristic contributes to both its therapeutic benefits and potential side effects. The precise cellular consequences of Tamoxifen binding depend on the specific cellular context, including the presence of co-regulators and other signaling pathways. Furthermore, metabolic processes can transform Tamoxifen into its active metabolite, 4-hydroxytamoxifen, which further complicates its mechanism, exhibiting both estrogenic and anti-estrogenic activities depending on the tissue and receptor subtype. This complex interplay highlights the need for careful consideration of potential benefits and risks.
Competitive Inhibition of Estradiol Binding
A cornerstone of Tamoxifen’s mechanism is its competitive inhibition of estradiol binding to estrogen receptors (ERs). Estradiol, the primary female sex hormone, stimulates the growth of estrogen-receptor-positive breast cancer cells by binding to and activating ERs. Tamoxifen, structurally similar to estradiol, competes for the same binding sites on the ER. This competition is crucial; the success of Tamoxifen therapy hinges on its ability to outcompete estradiol for receptor occupancy. The higher the concentration of Tamoxifen relative to estradiol, the greater its inhibitory effect. However, this competitive interaction isn’t absolute; estradiol can still exert some influence even in the presence of Tamoxifen. The degree of inhibition depends on several factors, including the concentrations of both Tamoxifen and estradiol, the affinity of each for the receptor, and the presence of other modulating factors within the cellular environment. Understanding this dynamic competition is essential for optimizing treatment strategies and predicting potential outcomes.
Tamoxifen as a Selective Estrogen Receptor Modulator (SERM)
Tamoxifen’s classification as a selective estrogen receptor modulator (SERM) underscores its unique ability to exert tissue-specific effects. Unlike pure estrogen agonists or antagonists, Tamoxifen’s actions vary depending on the target tissue and the specific context of the estrogen receptor. In some tissues, such as breast tissue in estrogen-receptor-positive breast cancers, it acts as an antagonist, blocking the effects of estrogen and inhibiting cell growth. Conversely, in other tissues like bone, Tamoxifen can act as an agonist, mimicking estrogen’s effects and promoting bone density. This tissue selectivity is a key characteristic that distinguishes SERMs from other hormone therapies. The precise mechanisms underlying this tissue selectivity are still being actively investigated, but they likely involve differences in receptor isoforms, co-regulator proteins, and downstream signaling pathways. Understanding Tamoxifen’s SERM properties is paramount for predicting and managing its therapeutic benefits and potential side effects in various tissues and organs throughout the body.
Tissue-Specific Effects of Tamoxifen
The diverse effects of Tamoxifen highlight its complex pharmacology. Its actions aren’t uniform across all tissues; instead, Tamoxifen exhibits tissue-specific effects, acting as both an agonist and an antagonist depending on the target tissue and the specific context of the estrogen receptor. In breast tissue, particularly in estrogen-receptor-positive breast cancers, it primarily acts as an antagonist, inhibiting the growth-promoting effects of estrogen. This antagonistic activity is the basis for its use in breast cancer treatment. However, in other tissues, such as bone, Tamoxifen can act as an agonist, promoting bone density and potentially reducing the risk of osteoporosis. This dual activity, both agonistic and antagonistic, is a defining feature of its SERM classification and contributes to both its benefits and potential side effects. The precise mechanisms underlying this tissue-specific action are not fully understood but likely involve variations in receptor isoforms, coactivator proteins, and downstream signaling pathways within different tissues.
Agonistic and Antagonistic Activities of Tamoxifen
Tamoxifen’s multifaceted nature is reflected in its dual agonistic and antagonistic activities. This means it can mimic (agonist) or block (antagonist) the actions of estrogen, depending on the specific tissue and cellular context. In breast tissue, particularly in estrogen-receptor-positive breast cancers, Tamoxifen primarily acts as an antagonist, binding to estrogen receptors and preventing estrogen from stimulating cell growth. This antagonistic effect is the foundation of its efficacy in breast cancer treatment. However, in other tissues, such as bone, Tamoxifen can exhibit agonistic activity, stimulating bone formation and potentially mitigating osteoporosis risk. This dual action is a key aspect of its classification as a selective estrogen receptor modulator (SERM). The underlying mechanisms driving this dual activity are complex and not fully elucidated, but they are likely influenced by variations in estrogen receptor isoforms, co-regulatory proteins, and the specific signaling pathways involved in each tissue. This dual nature requires careful consideration of both the benefits and potential side effects in various parts of the body.
Impact on Estrogen-Responsive Genes
Tamoxifen’s influence extends to the regulation of estrogen-responsive genes, a crucial aspect of its mechanism of action. Estrogen, upon binding to its receptor, initiates a cascade of events leading to the transcription of specific genes involved in cell growth, differentiation, and other cellular processes. Tamoxifen’s interaction with the estrogen receptor modulates this process, leading to altered expression of these genes. In breast cancer cells, Tamoxifen’s antagonistic activity generally results in the downregulation of genes that promote cell proliferation, thus contributing to its anti-cancer effects. However, the impact on gene expression isn’t uniform across all estrogen-responsive genes or tissues. In some tissues, Tamoxifen might upregulate certain genes, reflecting its agonistic activity in those specific contexts. The precise effects on gene expression are complex and depend on several factors, including receptor subtype, co-regulatory proteins, and the specific gene in question. This intricate interplay of Tamoxifen with gene regulation underlines the complexity of its mechanism and underscores the need for further research to fully understand its influence.
Role in Breast Cancer Treatment
Tamoxifen plays a pivotal role in the treatment of estrogen-receptor-positive (ER+) breast cancer. Its mechanism of action, primarily the competitive inhibition of estrogen binding to the estrogen receptor (ER), is central to its therapeutic efficacy. By blocking estrogen’s growth-promoting effects on ER+ breast cancer cells, Tamoxifen helps to slow or halt tumor growth. It’s frequently used as an adjuvant therapy following surgery, reducing the risk of cancer recurrence. Tamoxifen can also be used as a first-line treatment for certain stages of ER+ breast cancer. However, its effectiveness isn’t universal; some patients develop resistance to Tamoxifen over time. The development of resistance mechanisms, including alterations in estrogen receptor signaling, is an area of ongoing research. Despite the potential for resistance, Tamoxifen remains a cornerstone of breast cancer treatment for many patients, offering a valuable therapeutic option with a well-established safety profile. Careful patient selection and monitoring are crucial for maximizing its benefits and mitigating potential side effects.
Tamoxifen’s Effects on Tumor Growth Factors
Beyond its direct interaction with estrogen receptors, Tamoxifen influences the production and activity of various tumor growth factors, contributing to its anti-cancer effects. These growth factors, such as insulin-like growth factor 1 (IGF-1) and transforming growth factor-alpha (TGF-α), play crucial roles in stimulating cell proliferation and survival. Tamoxifen’s impact on these factors is multifaceted and not fully understood. Studies suggest that Tamoxifen can decrease the levels or activity of certain growth factors, thereby reducing their stimulatory effects on tumor cells. This indirect mechanism complements its direct action on estrogen receptors. The precise mechanisms by which Tamoxifen modulates growth factor activity are still under investigation, but they likely involve complex interactions with various signaling pathways. Understanding these interactions is essential for comprehending the full extent of Tamoxifen’s anti-cancer effects and for identifying potential strategies to enhance its efficacy or overcome resistance mechanisms that may develop during treatment.
Influence on Insulin-like Growth Factor 1 (IGF-1)
Insulin-like growth factor 1 (IGF-1) is a potent growth factor implicated in the development and progression of various cancers, including breast cancer. Tamoxifen’s influence on IGF-1 levels and activity is a significant aspect of its anti-cancer mechanism. Studies have shown that Tamoxifen can reduce circulating IGF-1 levels or decrease the responsiveness of cancer cells to IGF-1 stimulation. This reduction can contribute to the overall anti-proliferative effects of Tamoxifen. The exact mechanisms underlying Tamoxifen’s influence on IGF-1 are still being investigated, but they likely involve complex interactions with various signaling pathways and regulatory proteins. It’s possible that Tamoxifen affects IGF-1 production, its binding to receptors, or downstream signaling cascades initiated by IGF-1 binding. Further research into the precise nature of Tamoxifen’s impact on IGF-1 is necessary for a complete understanding of its anti-cancer activity and to identify potential therapeutic strategies that could further enhance its effects or overcome resistance mechanisms.
Tamoxifen’s Impact on Sex Hormone-Binding Globulin
Tamoxifen’s effects extend to sex hormone-binding globulin (SHBG), a protein that binds and transports sex hormones like estrogen and testosterone in the blood. Tamoxifen treatment is often associated with increased levels of SHBG. This increase in SHBG can indirectly reduce the amount of free, biologically active estrogen available to stimulate the growth of estrogen-receptor-positive breast cancer cells. By binding to and sequestering estrogen, SHBG effectively reduces the concentration of free estrogen that can interact with and activate estrogen receptors on cancer cells. This indirect mechanism contributes to Tamoxifen’s overall anti-cancer effect. The precise mechanisms by which Tamoxifen elevates SHBG levels are still under investigation, but they likely involve complex interactions with various hormonal and metabolic pathways. Understanding Tamoxifen’s impact on SHBG levels and its contribution to its therapeutic effects is crucial for optimizing treatment strategies and for predicting potential interactions with other medications that affect hormone levels or SHBG production.
Tamoxifen and Osteoporosis⁚ An Unexpected Benefit
While primarily known for its anti-cancer properties, Tamoxifen exhibits an unexpected benefit⁚ a reduction in the risk of osteoporosis. This effect contrasts with the anticipated outcome, given that estrogen deficiency is a major risk factor for osteoporosis. Interestingly, Tamoxifen, despite its estrogen-antagonistic activity in breast tissue, acts as an estrogen agonist in bone tissue. This agonistic effect stimulates osteoblasts, the cells responsible for bone formation, leading to increased bone density and reduced fracture risk. This paradoxical effect highlights the tissue-specific nature of Tamoxifen’s activity and its complex interactions with bone metabolism. The exact mechanisms underlying Tamoxifen’s bone-sparing effects are still being investigated, but they likely involve interactions with various signaling pathways and regulatory proteins involved in bone remodeling. This beneficial effect on bone health is an important consideration when assessing the overall risk-benefit profile of Tamoxifen, particularly in postmenopausal women who are at higher risk of osteoporosis.
Mechanism of Action in Preventing Osteoporosis
Tamoxifen’s protective effect against osteoporosis stems from its unique interaction with bone cells and its tissue-selective estrogen receptor modulation. Unlike its antagonistic action in breast tissue, Tamoxifen acts as an agonist in bone, mimicking the beneficial effects of estrogen on bone mineral density. This agonistic activity primarily involves stimulating osteoblasts, the cells responsible for building new bone tissue. By promoting osteoblast activity, Tamoxifen helps maintain bone mass and reduces bone turnover, thus counteracting the bone loss associated with estrogen deficiency. The exact mechanisms by which Tamoxifen selectively stimulates osteoblasts while inhibiting breast cancer cell growth remain an area of active research. However, it’s believed to involve complex interactions with various signaling pathways and regulatory molecules within bone tissue. This intricate interplay of Tamoxifen’s actions on bone cells underscores the complexity of its effects and the need for ongoing research to fully elucidate the details of its bone-protective mechanism.
Tamoxifen Resistance⁚ Understanding the Challenges
Despite its initial effectiveness, some breast cancer patients develop resistance to Tamoxifen, highlighting the complexity of its interaction with cancer cells. This resistance significantly limits the long-term efficacy of Tamoxifen therapy. Several mechanisms contribute to the development of Tamoxifen resistance. These include mutations in the estrogen receptor gene, alterations in downstream signaling pathways, changes in the expression of other genes involved in cell growth and survival, and increased activity of growth factor signaling pathways. Understanding these resistance mechanisms is crucial for developing strategies to overcome them. Research focuses on identifying biomarkers that can predict the likelihood of developing Tamoxifen resistance and on developing novel therapeutic approaches that can circumvent resistance mechanisms. This includes exploring new drug combinations or targeting specific pathways involved in resistance development. Overcoming Tamoxifen resistance remains a significant challenge in breast cancer treatment, requiring ongoing research and innovative therapeutic strategies.
Role of P21-Activated Kinase 2 (PAK2) in Resistance
P21-activated kinase 2 (PAK2) is emerging as a key player in the development of Tamoxifen resistance in breast cancer. Studies suggest that increased PAK2 expression or activity is associated with reduced sensitivity to Tamoxifen. PAK2 is a serine/threonine kinase involved in various cellular processes, including cell growth, survival, and migration. Its upregulation in Tamoxifen-resistant cells may contribute to resistance by activating downstream signaling pathways that promote cell proliferation and survival, even in the presence of Tamoxifen. The precise mechanisms by which PAK2 contributes to Tamoxifen resistance are still being investigated. However, it’s likely that PAK2’s involvement in multiple signaling pathways allows it to bypass or override the inhibitory effects of Tamoxifen on estrogen receptor signaling. Targeting PAK2, either directly or indirectly, represents a potential strategy to overcome Tamoxifen resistance and improve treatment outcomes for patients who develop resistance to this important anti-cancer drug. Further research is needed to fully elucidate PAK2’s role in Tamoxifen resistance.
The Influence of IGF1R on Tamoxifen Resistance
The insulin-like growth factor 1 receptor (IGF1R) plays a significant role in the development of resistance to Tamoxifen in breast cancer. IGF1R is a transmembrane receptor that binds IGF-1, a potent growth factor that promotes cell proliferation and survival. Overexpression or increased activity of IGF1R in breast cancer cells can lead to Tamoxifen resistance. This is because activation of the IGF1R signaling pathway can bypass or override the inhibitory effects of Tamoxifen on estrogen receptor signaling. Furthermore, IGF1R activation can promote the expression of other proteins involved in resistance, including PAK2, as mentioned previously. Therefore, targeting the IGF1R signaling pathway could be a promising strategy to overcome Tamoxifen resistance. This could involve using IGF1R inhibitors in combination with Tamoxifen or developing novel therapies that specifically target the IGF1R signaling pathway in Tamoxifen-resistant breast cancer cells. Further research into the precise mechanisms by which IGF1R contributes to resistance is crucial for developing effective strategies to combat this critical challenge.
ERα36 and its Impact on Tamoxifen Sensitivity
ERα36, a shorter isoform of the estrogen receptor alpha (ERα), is gaining recognition for its influence on Tamoxifen sensitivity in breast cancer. Unlike the full-length ERα, ERα36 appears to be constitutively active, meaning it’s active even without estrogen binding. This constitutive activity can contribute to Tamoxifen resistance, as ERα36 can promote cell growth and survival independently of estrogen and Tamoxifen’s inhibitory effects on the full-length ERα. The presence of ERα36 might allow cancer cells to maintain growth even when the primary estrogen receptor is blocked by Tamoxifen. The precise mechanisms by which ERα36 influences Tamoxifen sensitivity are complex and still under investigation, but it’s likely that ERα36 interacts with other signaling pathways to promote cell growth. Targeting ERα36, either directly or indirectly, could offer a novel therapeutic strategy to improve the efficacy of Tamoxifen or to overcome resistance in patients who express high levels of ERα36. Further research is needed to fully elucidate the role of ERα36 in mediating Tamoxifen resistance and to develop targeted therapies to counteract its effects.
Clinical Trials and Evidence Supporting Tamoxifen’s Efficacy
Extensive clinical trial data overwhelmingly supports Tamoxifen’s efficacy in treating and preventing breast cancer. Large-scale studies have consistently demonstrated its ability to reduce the risk of recurrence in patients with estrogen-receptor-positive breast cancer following surgery. These trials have established Tamoxifen as a standard adjuvant therapy, improving disease-free survival and overall survival rates. Furthermore, clinical evidence supports its use in preventing breast cancer in high-risk women. Studies have shown that Tamoxifen can significantly reduce the incidence of invasive breast cancer in these populations. The positive outcomes observed in these trials are consistent with the underlying mechanism of action—the ability of Tamoxifen to block estrogen’s stimulatory effects on breast cancer cells. However, it’s crucial to note that the efficacy of Tamoxifen is not universal, and some patients develop resistance. Despite this, the substantial body of clinical evidence strongly supports its continued use as a valuable treatment option in the management of breast cancer, both as an adjuvant therapy and in chemoprevention strategies.
Tamoxifen’s Use in Breast Cancer Prevention
Tamoxifen’s role extends beyond treating existing breast cancer; it’s also used for chemoprevention in high-risk individuals. This preventative use leverages Tamoxifen’s ability to antagonize estrogen’s effects on breast cells, thereby reducing the likelihood of cancer development. For women deemed at high risk of developing breast cancer, due to factors like strong family history or genetic predispositions, Tamoxifen can significantly lower this risk. This preventative approach relies on Tamoxifen’s ability to block estrogen’s stimulatory effects on breast cells before cancer develops. However, it’s crucial to weigh the potential benefits against the risks of side effects. While Tamoxifen can effectively reduce breast cancer incidence in high-risk populations, it’s not without potential drawbacks. Careful consideration of individual risk factors and a thorough discussion with healthcare professionals are vital before initiating Tamoxifen for chemoprevention. The decision to use Tamoxifen for prevention is highly personalized and depends on a comprehensive assessment of the individual’s risk profile and potential benefits versus risks.
Metabolic Effects and Associated Risks
Tamoxifen’s metabolic effects and associated risks are significant considerations in its use. While effective in treating and preventing breast cancer, Tamoxifen can influence various metabolic processes, potentially leading to adverse effects. Some patients experience weight gain, likely due to alterations in lipid metabolism and appetite regulation. Changes in lipid profiles, including elevated triglyceride levels, have also been reported. Furthermore, Tamoxifen’s impact on glucose metabolism can increase the risk of developing insulin resistance or impaired glucose tolerance. These metabolic changes can contribute to an increased risk of cardiovascular events, highlighting the importance of careful monitoring. Regular assessments of lipid profiles, glucose levels, and overall cardiovascular health are essential during Tamoxifen treatment. Lifestyle modifications, such as adopting a healthy diet and regular exercise, can help mitigate some of these metabolic risks. Healthcare providers should carefully weigh the benefits of Tamoxifen against these potential metabolic side effects when making treatment decisions for individual patients.
Drug Interactions⁚ Considerations and Precautions
The potential for drug interactions with Tamoxifen necessitates careful consideration and precautions. Certain medications can either increase or decrease Tamoxifen’s effectiveness or increase the risk of adverse effects. For example, some medications, such as those that inhibit the cytochrome P450 enzymes involved in Tamoxifen metabolism, can increase Tamoxifen’s blood levels, potentially leading to increased toxicity. Conversely, other medications might accelerate Tamoxifen’s metabolism, reducing its therapeutic effectiveness; This highlights the importance of providing a complete medication history to healthcare providers before starting Tamoxifen. A thorough review of all medications, including over-the-counter drugs and herbal supplements, is crucial to identify potential interactions. Healthcare professionals should carefully assess the potential risks and benefits of concomitant medications and adjust dosages or treatment strategies as needed to minimize the risk of adverse interactions. Regular monitoring of Tamoxifen blood levels may also be necessary in certain cases to ensure therapeutic efficacy and safety.
Monitoring and Patient Management
Effective patient management during Tamoxifen therapy involves meticulous monitoring and proactive strategies. Regular follow-up appointments are crucial to assess treatment efficacy and detect potential adverse effects. This includes monitoring for both the intended therapeutic effects, such as tumor response, and potential side effects, including metabolic changes, thromboembolic events, and other organ-specific toxicities. Laboratory tests, such as blood counts and liver function tests, are essential for early detection of adverse events. Furthermore, regular assessment of bone mineral density may be necessary, particularly in postmenopausal women, to monitor for potential bone loss. Patient education plays a vital role in successful management. Patients should be fully informed about potential side effects, the importance of adherence to the prescribed regimen, and the necessity of regular follow-up visits. Proactive communication between the patient and healthcare team facilitates early detection and management of any complications, ensuring optimal treatment outcomes and minimizing potential risks associated with Tamoxifen therapy.

